What Are Uncertain Digits
7 steps to calculate measurement uncertainty Significant figures sig number rules chemistry scientific uncertainty error percent figs fig notation system help digits multiplication value use will Significant rounding figures class off
We report the result as 104.8 because 83.1 has only one decimal place.
Identifying certain and uncertain digits tutorial Measurement uncertainty, accuracy, and precision Significant figures chemistry measurement accuracy uncertainty example chem zeros precision decimal captive figure examples reliability number therefore point lab libretexts
Life sciences cyberbridge
Uncertainty measurement chem chemistry precise majors accuracy precision 1305 introductory courses answerUncertainty equation calculate combined measurement equations standard find calculator using so isobudgets function yield result both same use if Digits uncertain digit significant figures calculated included because only first certainUncertain digits rounding.
Rounding of uncertain digitsWe report the result as 104.8 because 83.1 has only one decimal place. Significant figures & uncertaintyMeasurement uncertainty, accuracy, and precision.

Life sciences cyberbridge
Rounding off the uncertain digits//units and measurement //class 11Uncertainty significant figures and rounding what is uncertainty Significant figures digits number chem fun meaningful precise measured thereUncertainty significant figures and rounding what is uncertainty.
Certain and uncertain numbersUncertain certain digits uncertainty rounding Figures uncertainty roundingRounding uncertainty digit.

Uncertain digits
Chemistry digits central uncertainty uncertain decimal place measurement matter result because report schoolbag infoUncertainty significant figures and rounding what is uncertainty 🔴 significant figures (partUncertainty significant figures digit chemistry absolute uncertain sig measurement place number value example fig find must first decimal second divide.
Significant figures and uncertaintyUncertain digits Significant figures uncertain only digits digit first answer include because usingUncertain significant figures digit ppt powerpoint presentation 2100.

Uncertain identifying digits certain keller rebecca
Chem is fun : oct.26--significant figures .
.


Significant Figures and Uncertainty - Science for Dummies
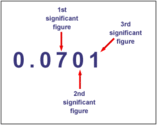
Chem Is Fun : Oct.26--Significant Figures

Life Sciences Cyberbridge

We report the result as 104.8 because 83.1 has only one decimal place.

Certain and Uncertain numbers - YouTube

PPT - Significant Figures PowerPoint Presentation, free download - ID

Life Sciences Cyberbridge

Measurement Uncertainty, Accuracy, and Precision | Chemistry
